Think of it as the volcano science experiment that children do with vinegar and baking soda. What is the cost of lithium ion battery.
 How We Made The Li Ion Rechargeable Battery Nature Electronics
How We Made The Li Ion Rechargeable Battery Nature Electronics
I think this is aluminum.
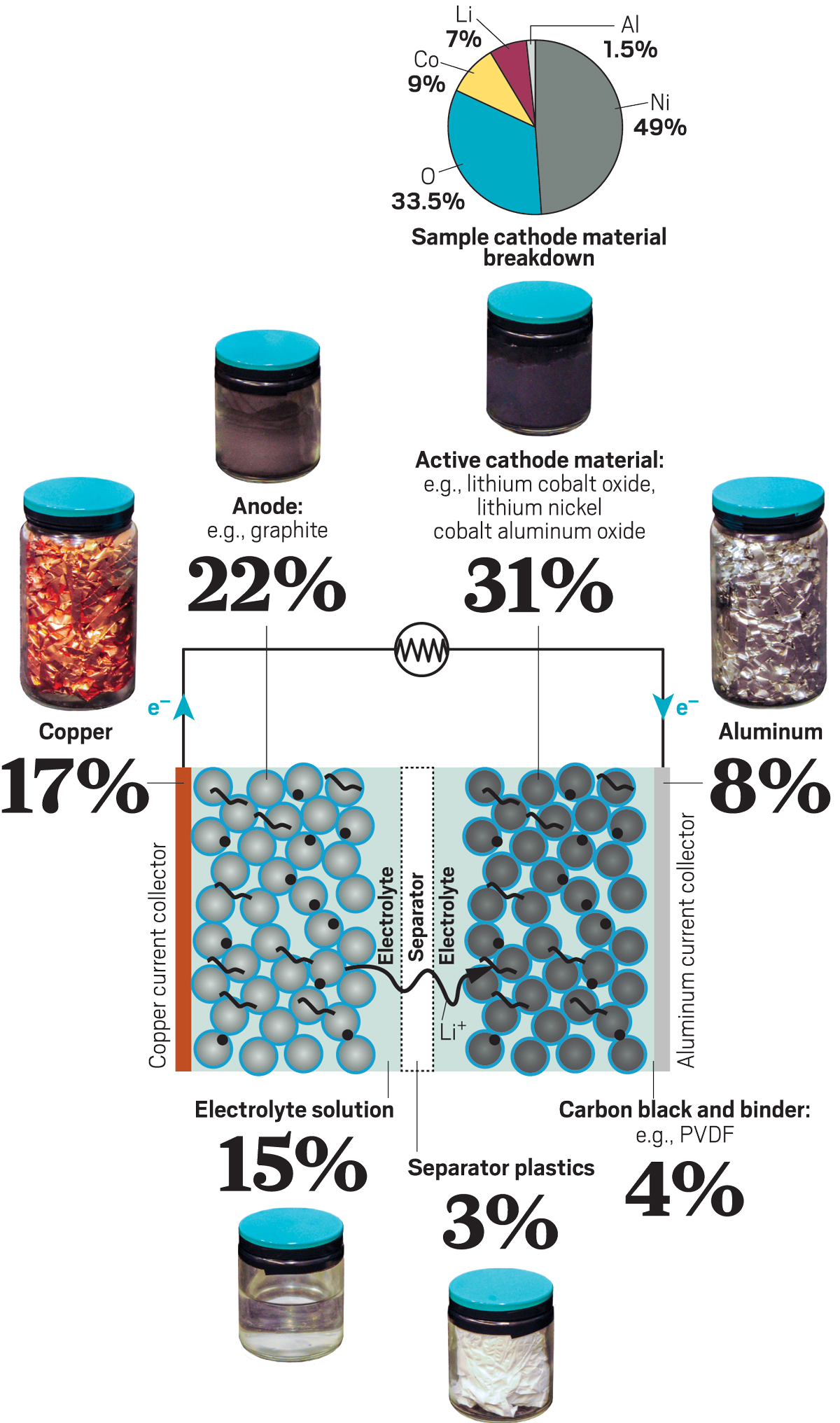
How do they make lithium batteries. Over 200 years ago Alessandro Volta invented the first battery. Battery production uses a lot of energy from the extraction of raw materials to the electricity consumed in manufacture. Processing of Lithium Ore The lithium extraction process uses a lot of waterapproximately 500000 gallons per metric ton of lithium.
The anode and cathode store the lithium. You can also charge your lithium battery using your boats outboard or inboard engine. A lithium ion battery is a type of rechargeable battery commonly used in laptops and cell phones.
The short answer is that a number of rare metals need to be dug out of the earth from various mines. Just to build each car batteryweighing upwards of 500 kilograms 1100 pounds in size for sport-utility vehicleswould emit up to 74 more C02 than producing an efficient conventional car if its made in. When you add the vinegar to the baking soda a chemical reaction happens causing bubbles.
Lithium ion batteries are manufactured in sets of electrodes and then assembled in cells. Manufacturing Process of Lithium batteries. How To Pick the Right Lithium Battery.
This is mainly because of its battery. So how exactly are these lithium-ion batteries for electric cars made. He discovered that by placing copper and zinc discs on top of each other and separating each with a brine soaked cloth he could create an electrical power source.
Lithium obtained from salars is recovered in the form of lithium carbonate the raw material used in lithium ion batteries. Brine extracting of lithium. Active material is mixed with polymer binders conductive additives and solvents to form a slurry that is then coated on a current collector foil and dried to remove the solvent and create a porous electrode coating.
It is then recommended that you install a DC-DC charger between your engines starter battery and the lithium battery to make sure its correctly charged. When you get to bigger sizes that can run at their nominal 37 volts like an 18650 the improvement over other battery types is significant. The findings among the more bearish ones around show that while electric cars are emission-free on the road they still discharge a lot of the carbon-dioxide that conventional cars do.
A thin layer of the slurry of carbon and lithium material is applied in large square swaths onto the metal terminal material. A lithium battery is formed of four key components. To extract lithium miners drill a hole in salt flats and pump salty mineral-rich brine to the surface.
The material of anode is Carbon based and the cathode is a Lithium metal oxide. Large rolls of raw materials are loaded into the coating machine. There is no single lithium ion battery.
The anode enables the electric current to flow through an external circuit and when the battery is charged lithium ions are stored in the anode. In Australia raw producers concentrate on the more energy-intensive and costlier hard rock. These are then packaged into small individual battery cells alongside other materials such as plastic aluminum and steel before themselves being packed into battery modules.
The end result is a battery pack which is made up of multiple battery. The movement of the lithium ions creates free electrons in the anode which creates a charge at the. This reduction in space for electrochemical storage is why they do not really outperform Ni-MH right now.
A battery is made up of an anode cathode separator electrolyte and two current collectors positive and negative. They are delivered as a black powder and are stored with great care. As such making a AA sized li-ion battery requires sacrificing space in the battery for electronics to change the voltage and keep the battery safe.
The material that will be coated on to the anode and cathode is mixed. The electrolyte carries positively charged lithium ions from the anode to the cathode and vice versa through the separator. The raw lithium is then processed into lithium chloride to be used in applications like batteries.
Most lithium is commercially produced from either the extraction of lithium-containing salts from underground brine reservoirs or the mining of lithium-containing rock such as spodumene. His invention was called the Voltaic Pile in honour of the inventor and because it was well a pile of materials. To create power lithium ions move from the negative electrode through an electrolyte to the positive electrode.
The production process is fairly straightforward and requires only natural evaporation which leaves behind not only lithium but also magnesium calcium sodium and potassium. Lithium production from clay sources is expected to become commercially viable though perhaps not. Connecting the two plates together with a wire causes a chemical reaction in the battery which makes electrons flow from one plate to the other.
The anode and cathode in the Lithium batteries are of the same form and act as a current collector and conducts the current in and out of the cell. Charging Lithium Batteries With an Engine Alternator. It has the cathode which determines the capacity and voltage of the battery and is the source of the lithium ions.
The bigger the electric car and its range the more battery cells are needed to power it and consequently the more carbon produced.







/pogo-online-games-site-6dee6691ec904f2cad019379e9c750bc.png)


